Original Article
Evaluation of predictive factors associated with prognosis of patients with Solitary Hepatocellular Carcinoma receiving transcatheter Arterial Chemoembolization
Shou-Wu Lee,1,3,4,7 Yu-Chi Cheng,3,4 Chieh-Ling Yen,1 Sheng Shun Yang1,3,4,5,6,7 Teng-Yu Lee,1,3
1Division of Gastroenterology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
2Department of Radiology, Taipei Veterans General Hospital, Taipei, Taiwan
3Department of Internal Medicine, Chung Shan Medical University, Taichung, Taiwan
4Department of Internal Medicine, Yang Ming Chiao Tung University, Taipei, Taiwan.
5Ph.D. Program in Translational Medicine, Chung Hsing University, Taichung, Taiwan
6Institute of Biomedical Sciences, Chung Hsing University, Taichung, Taiwan
7Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University
Received Date: 21/06/2022; Published Date: 06/07/2022.
*Corresponding author: Shou-Wu Lee, Division of Hepatology and Gastroenterology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung 1650 Taiwan Boulevard, Sec. 4, Taichung 40705, Taiwan.
DOI: 10.55920/IJCIMR.2022.02.001082
Abstract
Aim: Transcatheter arterial chemoembolization (TACE) is the recommended treatment modality for intermediate stage hepatocellular carcinoma (HCC). The aim of this study was to evaluate the predictive factors associated with prognosis of patients with solitary HCC receiving TACE.
Methods: Patients with solitary HCC were collected at Taichung Veterans General Hospital from January 2005 to December 2009. Patients with or without complete response (CR) were assigned to the CR group or the non-CR group according to mRECIST criteria. The clinical and radiological characteristics of the two groups were compared.
Results: Among a total of 84 enrolled patients, there were 38 (45.2%) and 46 (54.8%) patients in the CR group and non-CR group, respectively. The CR group had significantly smaller tumor size (mean 3.76cm vs. 8.59cm, p=0.001), and a higher rate of no residual tumor blush (RTB) (94.7% vs. 56.5%, p=0.001), compared with the non-CR group. Logistic analysis showed the HCC size (OR 0.66, 95% CI 0.54-0.81, p=0.001) and no RTB (OR 6.01, 95% CI 1.11-33.28, p=0.038) each had a significant impacts on whether CR was achieved with TACE. Better overall survival was found in patients with CR who underwent TACE, had no RTB, or had small-sized HCC.
Conclusion: Small-sized tumor and no RTB were associated with a favorable radiological tumor response in patients with solitary HCC receiving TACE.
Keywords: Hepatocellular carcinoma; radiological characteristics; transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) occurs most often in underlying chronic liver diseases including advanced fibrosis or cirrhosis, which is usually related to chronic hepatitis B virus (HBV), hepatitis C virus (HCV) infection, prolonged alcohol use, or nonalcoholic fatty liver disease.1.2 The Barcelona Clinic Liver Cancer (BCLC) staging system can be used to assess therapeutic options,3 such as transcatheter arterial chemoembolization (TACE), which is recommended as the treatment modality for intermediate stage, or BCLC stage B, HCC.4 For solitary HCC, curative treatments such as hepatic resection, liver transplantation, and radiofrequency ablation (RFA) are the usual recommended treatment modalities. However, these modalities sometimes cannot be applied in all patients with solitary HCC for various reasons unique to individual cases.
According to previous reports, TACE slows cancer progression and improves survival, compared to those treated with best supportive care, in patients with unresectable HCC.5,6 In addition, some clinical studies have evaluated patients with solitary HCC undergoing TACE.7,8 However, the reported therapeutic outcomes among these patients vary.
The aim of our study was to evaluate the predictive factors associated with the prognosis of patients with solitary HCC receiving TACE as the primary treatment.
Methods
HCC patients who were newly diagnosed in accordance with the AASLD guidelines9 were collected at Taichung Veterans General Hospital from January 2005 to December 2009. The enrolment criteria were a diagnosis of solitary HCC and use of TACE as the primary treatment. These subjects were not eligible for hepatic resection, liver transplantation or RFA due to individual reasons, such as multiple comorbidities, no suitable donor, risks of postopertative hepatic dysfunction or tumors in locations that are difficulty to approach. The exclusion criteria included diagnosis with multiple HCCs, appearance of tumor with major vascular invasion or extrahepatic spread, cirrhotic Child-Pugh stage C, poor performance status or lost to of follow-up within the following day.
The clinical parameters of all enrolled patients, including age, gender, and liver function, such as total bilirubin, alanine aminotransferase (ALT), and alpha-fetoprotein (AFP), as well as presence of chronic HBV and HCV infection, cirrhotic Child-Pugh stage, and tumor size were collected.
The radiological findings were recorded. For superselective TACE, the tip of the catheter was placed into the hepatic arterial branch afferent to the segment or subsegment sites where the tumor was located. In non-selective TACE, a lobar technique was carried out in the case of a nodule fed by multiple arteries. On the post-TACE angiogram, tumor-feeding artery blockage was defined according to whether or not tumor-supplying arteries could be visualized. Presence of residual tumor blush (RTB) was defined as either unchanged tumor stain or a reduction in intensity or size compared with the pre-TACE image. According to post-TACE CT images, depending on the pattern of tumor covered by lipiodol, complete lipiodol retention was defined as more than 90 % lipiodol retention and no peripheral filling defects..
Patients were assessed every 2 months by dynamic imaging study until the endpoints were reached, including death, disease progression, or treatment failure after TACE. The assessment of the best tumor response was done according to the modified RECIST (mRECIST) criteria10 with four response categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The patients with CR were categorized into the complete response (CR) group and others were categorized into the non-complete response (non-CR) group.
Statistical analysis
Data are expressed as standard deviation of the mean for each of the measured parameters. The positive rates of each stratified group are both expressed as a percentage of the total patient number. Statistical comparisons were made using Pearson’s chi-square test or Fisher's exact test in order to compare the effects of the positive rate of each stratified group. Independent t test was used to analyze continuous variables. A p-value below 0.05 was considered statistically significant. Hazard ratios (HR) with a 95% confidence interval (CI) were calculated by multivariate Cox’s regression to examine the strength of association between the radiological variables and the responses following TACE. Survival analysis was carried out using the Kaplan-Meier method for analysis and comparisons were subsequently performed with the log-rank test.
Results
A total of 84 patients were enrolled, and the characteristics of these cases are displayed in Table 1. The median age was 70.11 years, and male predominance (78.6%) was noted. Thirty-six patients (42.9%) and 31 patients (36.9%) had chronic HBV and HCV infection. Fifty-eight cases (69.0%) and 26 cases (31.0%) belonged to Child-Pugh stages A and B, respectively. The median HCC diameter was 6.46 cm, and there were 22 cases (23.8%), 20 cases (26.2%) and 42 cases (50.0%) with HCC ≤2cm (BCLC stage 0), HCC >2cm and ≤5cm (BCLC stage A) and HCC >5cm (BCLC stage B), respectively.
The outcomes of the enrolled patients after TACE are also listed in Table 1. The numbers of cases with CR, PR, SD, and PD were 38 (45.2%), 20 (23.8%), 13 (15.5%), and 13 (15.5%) respectively. Among patients who received TACE, the objective response rate (ORR) was 69.0%, and the disease control rate (DCR) was 84.5%. Overall, there were 38 patients (45.2%) in the CR group and 46 (54.8%) patients in the non-CR group. The overall survival of the enrolled patients was 23.77±12.72months, and the one-, two- and three-year survival rates were 71.4%, 53.6% and 42.9%, respectively.
Table 1: The general data and outcomes of the patients with TACE
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine amino- transferase; CR, complete response; DCR, disease control rate; HBV, hepatitis B; HCV, hepatitis C; M, mean; N, number of pa- tients; OR, objective response; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable dis- ease; SD, standard derivation.
The characteristics of the non-CR group and the CR group are displayed in Table 2. Age, gender, rate of chronic viral hepatitis infection, Child-Pugh stage, and laboratory parameters, including total bilirubin, ALT, and AFP in the two groups were similar. The patients with ≤2cm HCC had the highest rate of CR (18 of all 22 cases, 81.8%), followed by those with >2cm and ≤5cm HCC (11 of all 20 cases, 55.0%), and those with >5cm HCC (9 of all 42 cases, 21.4%). The difference was significant (p=0.001). Similarly, the CR group had a significantly smaller tumor size than that of the non-CR group did (mean HCC size 3.76cm vs. 8.59cm, p=0.001).
A comparison of the positive rates of radiological characteristics in the non-CR group and the CR group is also shown in Table 2. The CR group had an non-significantly lower rate of super-selection for TACE (73.7% vs. 82.6%, p=0.321), non-significantly higher rates of complete lipiodol retention (81.6% vs.71.7%, p=0.292) and tumor feeding artery blockage (71.1% vs. 60.9%, p=0.329), but a significantly higher rate of no RTB (94.7% vs. 56.5%, p=0.001), compared with the non-CR group.
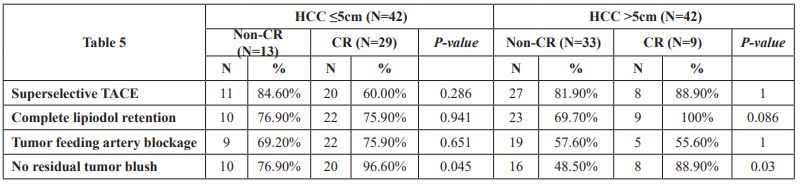
Further analysis of the association between radiological characteristics and complete tumor response with TACE according to the HCC size (HCC ≤5cm or >5cm) is shown in Table 3. Similarly, the image finding of no RTB still had a significant positive impact both in the subgroup of small-sized HCC (96.6% vs. 76.9%, p=0.045) and in the subgroup of large-sized HCC (88.9% vs. 48.5%, p=0.030). The prevalence of complete lipiodol retention was higher in the patients with HCC >5cm and CR than in those with non-CR, although the difference was non-significant (100% vs. 69.7% p=0.086).
Table 2: The general data and radiological characteristics of the non-CR group and the CR group
P-values were analyzed with independent t testa; Pearson’s Chi-square testb; Fisher’s exact testc
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; HBV, hepatitis B; CR, complete response; HCV, hepatitis C; M, mean; N, number of patients; SD, standard derivation; TACE, transcatheter arterial chemoembolization. # The rate of CR in HCC size ≤2cm: 81.8% (18/22), >2cm and ≤5cm: 55.0% (11/20), >5cm: 21.4% (9/42).
Table 3: The radiological characteristics between the subgroup of HCC≤5cm or >5cm
P-values were analyzed Fisher’s exact test Abbreviations: CR, complete response; N, number of patients; TACE, transcatheter arterial chemoembolization
Logistic analysis of the patients who achieved complete tumor response with TACE is shown in Table 4. The clinical parameters including age, gender, viral hepatitis, Child-Pugh stage, AFP, and TACE image characteristics, including superselective TACE, complete lipiodol retention, and tumor feeding artery blockage, had non-significant effects. In contrast, the HCC size (OR 0.66, 95% CI 0.54-0.81, p=0.001) had a significant negative impact, and the TACE image of no RTB (OR 6.01, 95% CI 1.11-33.28, p=0.038) had a significant positive effect on achievement of complete tumor response after TACE.
The overall survival between the non-CR group and the CR group, between patients with and without RTB, and among subjects with different HCC sizes are displayed in Figures 1, 2, and 3, respectively. The patients in the CR group had a significantly longer overall survival than those in the non-CR group (31.45±8.05 months vs. 22.74±12.17 months, p=0.002). The subjects with TACE image of no RTB had a significantly better overall survival than those without (26.69±11.98 months vs. 15.57±11.28 months, p=0.001). The patients with HCC >5cm had a significantly worse outcome of overall survival than those with HCC >2cm and ≤5cm or HCC ≤2cm (19.61±13.08 months vs. 28.20±11.56 months vs. 27.70±10.74, p=0.015).
Table 4: Logistic analysis of the achievement of complete tumor response with TACE.
Analyzed with Logistic regression adjusted with age, gender, viral hepatitis, cirrhotic Child-Pugh stage and AFP Abbreviations: AFP, alpha-fetoprotein; CI, confidence interval; HCC, hepatocellular carcinoma; N, number of patients; OR, oddi ra- tio; TACE, transcatheter arterial chemoembolization.
DISCUSSION
HCC is one of the most types of malignancy worldwide, and less than half of patients are diagnosed at an early stage.11 Recommended treatment modalities for resectable HCC are curative treatments including hepatic resection, liver transplantation, and RFA. The treatment modalities for unresectable HCC are palliative treatments, such as TACE or systemic therapy.3 However, these curative modalities may not be suitable for the treatment of solitary HCC in some cases with a variety of reasons unique to individual patients. Hence, TACE was adapted to meet the needs of such patients based on clinical judgment.
The modified RECIST is widely used for assessing the radiological response of HCC following TACE.10 Determination of treatment response following TACE by measuring residual viable tumor tissue has been proven to be a surrogate marker of overall survival.12,13 Our results found the radiological CR occurred in 38 cases of the 84 patients (45.2%) enrolled in this study. Clinical parameters, including age, gender, rates of chronic viral hepatitis infection, Child-Pugh stage, and laboratory parameters, had no impact on the achievement of CR among these patients.
One retrospective Korean study, which enrolled 175 patients with solitary HCC receiving TACE, noted the rate of CR was 68% in all cases and tumor size ≤3 cm was also a significant predictor according to the results of multivariate analysis (OR 2.024, 95% CI 1.295-6.135, P=0.049).7 A retrospective study conducted in Italy, which investigated 148 patients with solitary HCC who underwent TACE, found the overall rate of CR was 64%, and smaller tumor diameter was a significant predictor of achievement of radiological CR. (≤3cm HCC vs. >3 cm HCC: 73% vs. 54%, p=0.017).8 Our study had a similar result. The patients with a small-sized HCC (≤2cm) had a higher rate of CR than those with a larger-sized HCC (>2cm and ≤5cm or >5cm) (81.8% vs. 55.0% vs. 21.4%, p=0.001).
TACE involves the intra-arterial infusion of a cytotoxic drug and embolization of the blood vessel using gelatin sponge particles or microspheres. The difference in TACE efficacy according to the HCC size might be explained by the presence of more poorly enhanced daughter nodules or satellite lesions around large-sized HCC, rendering it less likely to achieve CR in response to TACE.14 The another reason might be due to the fact that anticancer drugs easily pass through the arterioportal shunt that more in large-sized HCC.1
According to previous studies, the characteristics of lipiodol retention by tumor tissue could be considered a prognostic marker,15 and incomplete lipiodol pattern was correlated with a higher risk of recurrence.16,17 One US retrospective study enrolled 105 patients with unresectable HCC who underwent TACE, and results showed the endpoints of no RTB and reduced antegrade arterial flow were independent positive prognostic indicator of survival.18
Our results found the radiological characteristic of no RTB was a significant predictor of achievement of radiological CR with TACE in patients with solitary HCC (both small- and large-sized tumors). Other radiological characteristics, including super-selection for TACE, complete lipiodol retention and tumor feeding artery blockage, were not associated with the radiological tumor responses of the subjects with solitary HCC receiving TACE.
A radiological tumor response to TACE has been identified as an independent prognostic factor that is capable of predicting outcomes such as overall survival in these patients.12,19 A previous study conducted in Italy reported average overall survival durations of 37 months and 28 months in patients with CR and non-CR after TACE, and the difference was significant (p=0.048).7 Our study also found a significant better overall survival in the CR group than that in the non-CR group (31.45 months vs. 22.74 months, p=0.002). Moreover, smaller tumor size (27.70-28.20 months vs. 19.61 months, p=0.015) and no RTB (26.69 months vs. 15.57 month, p=0.001) were both positively correlated with prolonged overall survival.
Our study had a few limitations. First, this study design was retrospective, and selection or reporting bias may have existed. Second, epirubicin and lipidiol dosage of TACE were not recorded, and the dose-tumor response relationship was not assessed. Third, patient tolerability to TACE and operator-dependent endpoints were variable. Fourth, combined or subsequent alternative tumor therapies were not taken into consideration, and tumor recurrences after radiological CR were also not recorded. Further prospective studies that include a higher number of cases and more variables are necessary.
In conclusion, smaller tumor size and no RTB were associated with a favorable radiological tumor response in patients with solitary HCC receiving TACE.
Reference
- Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: Modalities, indication, and patient selection J Hepatol 2015;62:1187–95.
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–370.
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61–74.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Geschwind JFH, Ramsey DE, van der Wal BCH, et al. Transcatheter arterial chemoembolization of liver tumors: Effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Intervent Radiol 2003;26:111–7.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003; 37:429–42.
- Baek MY, Yoo JJ, Jeong SW, et al. Clinical outcomes of patients with a single hepatocellular carcinoma less than 5 cm treated with transarterial chemoembolization. Korean J Intern Med 2019;34:1223-1232.
- Terzi E, Piscaglia F, Forlani L, et al. TACE performed in patients with a single nodule of hepatocellular carcinoma. BMC Cancer 2014;14:601.
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55.
- Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 2011;55:1309–1316.
- Jung ES, Kim JH, Yoon EL, Lee HJ, Lee SJ, Suh SJ, et al. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Hepatol 2013;58:1181–1187.
- Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol 2015;7:2009-2019.
- Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699–704.
- Kinugasa H, Nouso K, Takeuchi Y, et al. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterol. 2012;47:421–6.
- Marco Dioguardi Burgio, Riccardo Sartoris, Claudia Libotean, et al. Lipiodol retention pattern after TACE for HCC is a predictor for local progression in lesions with complete response. Cancer Imaging 2019;19:75.
- Jin B, Wang D, Lewandowski RJ, Riaz A, Ryu RK, Sato KT, Larson AC, Salem R, Omary RA. Chemoembolization endpoints: effect on survival among patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196:919-28
- Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304–10.